
- Age: 8+
- Time: 20
- (Setup: 5min, Activity: 10 min, Cleanup: 5 min)
- Materials: $8
In this mission, you’ll look at how atoms are put together inside a material, and how that affects its properties. You’ll do this by creating a model made of candy, which will show some of the same patterns a scientist would see with a (non-candy) microscope!
Download PDF-
what you need
WHAT YOU NEED
Materials:
- 1 cup small round candies, all one color (or small plastic BBs)
- ¼ cup large round candies, all one color (or larger plastic BBs)
Equipment:
- Large kitchen bowl
If you have extra candies or BBs, or more different sizes / colors, that's ok too - that just means you can do more experiments!
-
What To Do
WHAT TO DO
- Pour enough of the small candies into the bowl to cover the bottom with one layer of candy. Each small candy represents one small atom. Do you see any patterns?
- Gently shake the bowl from side to side and watch how the “atoms” move. Keep shaking until all of the atoms line up in straight lines. Do any of the rows go all the way from one side of the bowl to the other? What happens at the edges? Are there any gaps or cracks in the patterns you see?
- Add the larger candies—approximately ¼ the number of small candies. Each large candy represents one large atom. Use your hands to mix up the balls and distribute the larger ones throughout. Again, gently shake the mixture back and forth and watch what happens to the atoms. Can you get them to line up again? Where do the large atoms go? Do you get different outcomes depending on how hard to shake?
- Try more things! What happens if you add even more of the larger candies, or just a few? What if you add enough small candies to have more than one layer? What if you have lots of large candies and only a few small ones? What if you make a nice clear arrangement with gentle shaking and then shake harder? Or dump in all the candies at once and don’t shake at all? Or mix three different sizes, or two candies of the same size but different colors? Just like there are lots of different sizes and shapes and mixtures for round candies, there are lots of different ways that atoms can be mixed, with lots of different possibilities for what happens.
Clean-up:
You can munch on your candy or reuse the BBs for another purpose.
-
What's Happening?
 WHAT’S HAPPENING?
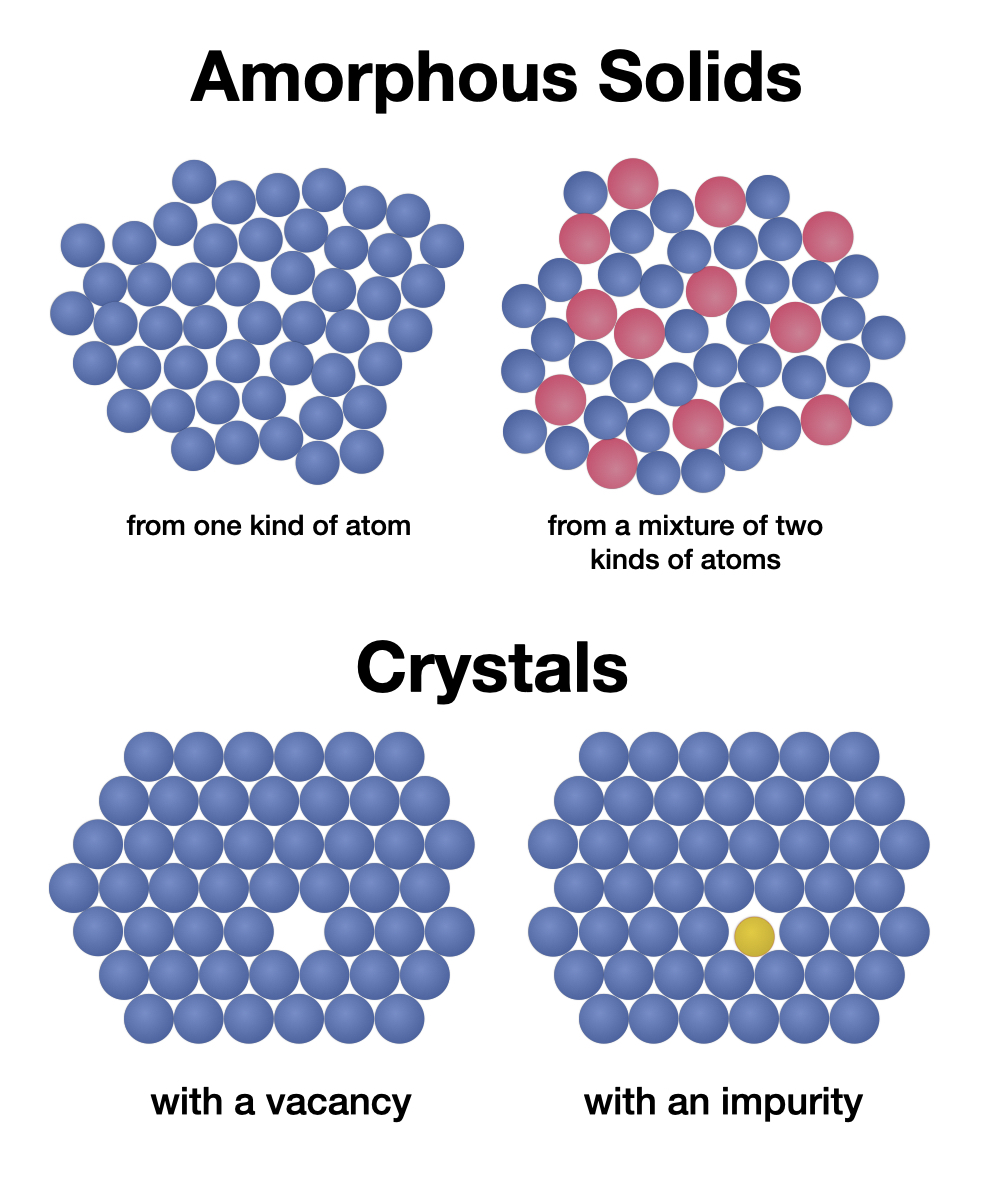
WHAT’S HAPPENING?Everything around us is made of tiny building blocks called atoms – so tiny a powerful microscope is needed to see them. Scientists create bigger models of things we can’t see to help us understand how those things work. The different candies or BBs that you used in this activity represent the atoms that make up all materials, and the model you made actually shows some of the same patterns and structures that you would see with a microscope.
Just as you added energy by gently shaking the bowl, materials scientists add energy (heat) to atoms to make them line up into an orderly structure. This is how atoms are arranged inside a typical metal. You may have noticed some boundaries where the lines of atoms don’t quite match up because the pattern on one side is shifted or turned compared to the other. These boundaries are the edges between different crystals in the metal— defects in the structure where the material might crack, bend, or corrode (rust). You might also see places where one atom is missing from the crystal pattern.
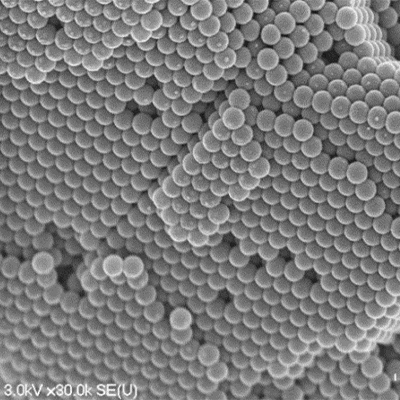
When you added the larger candies, you created a mixture similar to an alloy, a combination of metals that contains atoms of different sizes. The larger atoms might form their own crystal, or might mix into the smaller ones to break up the regular structure of the smaller atoms. You might even see a few smaller atoms sitting inside a crystal of larger atoms, or fitting into the gaps between crystals of the larger ones. If you add lots of candies, you might see them arrange in several layers, each layer fitting into the one underneath. The picture of the big bowl of BB’s shows some of these layers. These different arrangements of atoms can lead to some very different behaviors of the materials at a large scale.
-
So What?
 SO WHAT?
SO WHAT?Crystals have atoms arranged in a very ordered structure, like the balls at the bottom of the bowl after you gently shake it up. This is called a crystalline structure. Most crystals aren’t perfect: they might have a spot here or there that's missing its atom (a vacancy) or has the wrong kind of atom (an impurity). Sometimes two different crystals inside a material point in different directions and don’t match quite right where they touch. If lots of atoms of different sizes are mixed together and cooled quickly before then can get organized, they might form an amorphous structure with no crystal patterns at all.
Defects in crystals aren’t necessarily a bad thing—they actually determine how different materials behave in our daily lives. For example, impurity atoms at the boundaries between crystals can make a metal stronger, or more resistant to rusting. In certain alloys, different kinds of atoms take different places in the crystal and then lock each other into place. These “superalloys” are very resistant to bending – they are used to make the turbine blades in jet engines. Since the energy in a metal tends to be lost at the boundaries between crystals, an amorphous metal can actually be stronger and bouncier than a crystalline one. Scientists and engineers are still trying to figure out what amorphous metals might be good for!
Scientists observe the atomic crystal structure of materials to determine how the materials will behave in the world. They can look at materials up close—even at the atomic level—using a high-resolution electron microscope. However, using this technology is very expensive and takes a long time compared to using other techniques. This technology can also be dangerous; it uses a very high voltage and emits X-rays, so they are built with many safeguards in place.
When carbon atoms are arranged in a flat sheet, they form a crystal structure that looks like a honeycomb. This is called graphene. The picture of graphene shown here was taken by Sai Bachu, a student at Penn State University, using an electron microscope. The white line on the image is one billionth of a meter long (a nanometer). The crystal structure of graphene makes it extremely strong: the “carbon fibers” used in sports equipment and airplane wings are made from wrapped-up layers of graphene.
- Scientists In Action
-
For Teachers
For Teachers
Below are suggested alignment between this activity and concepts in the Next Generation Science Standards.
Performance Expectations
- 2-PS1-3: Make observations to construct an evidence-based account of how an object made of a small set of pieces can be disassembled and made into a new object.
- 5-PS1-4: Conduct an investigation to determine whether the mixing of two or more substances results in new substances.
- MS-PS1-1: Develop models to describe the atomic composition of simple molecules and extended structures.
Disciplinary Core Ideas:
PS1.A: Structure and Properties of Matter
2nd Grade
- A great variety of objects can be built up from a small set of pieces.
5th Grade
- Matter of any type can be subdivided into particles that are too small to see, but even then the matter still exists and can be detected by other means. A model showing that gases are made from matter particles that are too small to see and are moving freely around in space can explain many observations, including the inflation and shape of a balloon and the effects of air on larger particles or objects.
Middle School
- Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
- Solids may be formed from molecules, or they may be extended structures with repeating subunits (e.g., crystals)
PS1.B: Chemical Reactions
5th Grade
- When two or more different substances are mixed, a new substance with different properties may be formed.
Please click on the PDF below for a more detailed description of how this activity ties to NGSS
Download PDF


